Abstract
Introduction
Disease relapse after allogeneic stem cell transplantation is a major cause of treatment-related morbidity and mortality in patients with myeloproliferative neoplasms (MPNs). The cellular and molecular mechanisms for MPN relapse are not well understood. In this study, we investigated the role of cell competition between wild-type and JAK2V617F mutant cells in MPN disease relapse after stem cell transplantation.
Methods
JAK2V617F Flip-Flop (FF1) mice (which carry a Cre-inducible human JAK2V617F gene driven by the human JAK2 promoter) were crossed with Tie2-cre mice to express JAK2V617F specifically in all hematopoietic cells and vascular endothelial cells (Tie2FF1), so as to model the human diseases in which both the hematopoietic stem cells and endothelial cells harbor the mutation.
Results
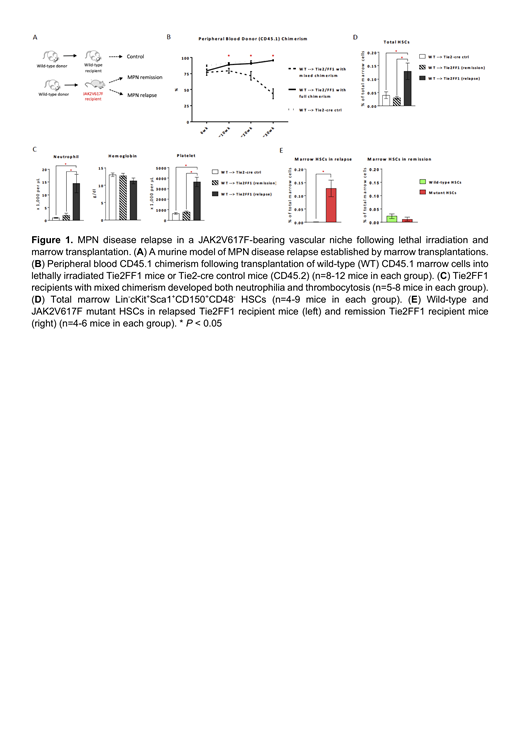
To investigate the underlying mechanisms for MPN disease relapse, we transplanted wild-type CD45.1 marrow directly into lethally irradiated Tie2FF1 mice or age-matched control mice(CD45.2). During a 6-7mo follow up, while all wild-type control recipients displayed full donor engraftment, ~60% Tie2FF1 recipient mice displayed recovery of the JAK2V617Fmutant hematopoiesis (mixed donor/recipient chimerism) 10 weeks after transplantation and developed a MPN phenotype with neutrophilia and thrombocytosis, results consistent with our previous report. Using CD45.1 as a marker for wild-type donor and CD45.2 for JAK2V617F mutant recipient cells, we found that the wild-type HSCs (Lin -cKit +Sca1 +CD150 +CD48 -) were severely suppressed and the JAK2V617F mutant HSCs were significantly expanded in the relapsed mice; in contrast, there was no significant difference between the wild-type and mutant HSC numbers in the remission mice. (Figure 1)
Cell competition is an evolutionarily conserved mechanism in which "fitter" cells out-compete their "less-fit" neighbors. We hypothesize that competition between the wild-type donor cells and JAK2V617F mutant recipient cells dictates the outcome of disease relapse versus remission after stem cell transplantation. To support this hypothesis, we found that there was no significant difference in cell proliferation, apoptosis, or senescence between wild-type and JAK2V617F mutant HSPCs in recipient mice who achieved disease remission; in contrast, in recipient mice who relapsed after the transplantation, wild-type HSPC functions were significantly impaired (i.e., decreased proliferation, increased apoptosis, and increased senescence), which could alter the competition between co-existing wild-type and mutant cells and lead to the outgrowth of the JAK2V617F mutant HSPCs and disease relapse. (Figure 2)
To understand how wild-type cells prevent the expansion of JAK2V617F mutant HSPCs, we established a murine model of wild-type and JAK2V617F mutant cell competition. In this model, when 100% JAK2V617F mutant marrow cells (from the Tie2FF1 mice) are transplanted alone into lethally irradiated wild-type recipients, the recipient mice develop a MPN phenotype ~4wks after transplantation; in contrast, when a 50-50 mix of mutant and wild-type marrow cells are transplanted together into the wild-type recipient mice, the JAK2V617F mutant donor cells engraft to a similar level as the wild-type donor cells and the recipient mice displayed normal blood counts during more than 4-months of follow up. In this model, compared to wild-type HSPCs, JAK2V617F mutant HSPCs generated significantly more T cells and less B cells in the spleen, and more myeloid-derived suppressor cells (MDSCs) in the marrow; in contrast, there was no difference in T, B, or MDSC numbers between recipients of wild-type HSPCs and recipients of mixed wild-type and JAK2V617F mutant HSPCs. We also found that program death ligand 1 (PD-L1) expression was significantly upregulated on JAK2V617F mutant HSPCs compared to wild-type cells, while PD-L1 expression on mutant HSPCs was significantly decreased when there was co-existing wild-type cell competition. These results indicate that competition between wild-type and JAK2V617F mutant cells can modulate the immune cell composition and PD-L1 expression induced by the JAK2V617F oncogene. (Figure 3)
Conclusion
Our study provides the important observations and mechanistic insights that cell competition between wild-type donor cells and JAK2V617F mutant recipient cells can prevent MPN disease relapse after stem cell transplantation.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal